
Neon Bohr Model — Diagram, Steps To Draw Techiescientist
If you want to make a model of a neon atom, you should keep in mind that it has 10 electrons. Spray paint your foam balls to differentiate what they represent. Separate them into three groups: the large foam ball, two of the small ones, and the remaining eight.

Pin on para Adrian
This Bohr-Rutherford model explains the structure of the atom, placement of different atomic species inside the atom as well as the charge on different atomic particles. It also explained why electrons remain confined to their shells instead of falling inside the nucleus.
Neon Electron Configuration Silicon Atom , Transparent Cartoon Jing.fm
The DVMS structure for a Slater-Jastrow model of the Neon atom is shown in Fig. 1. The individual r i are plotted as yellow or green spheres, with a translucent sphere centered on the nuclear position. The superimposed green and yellow spheres are the core electrons. That is to say, the average positions of the core electrons are at the nucleus.
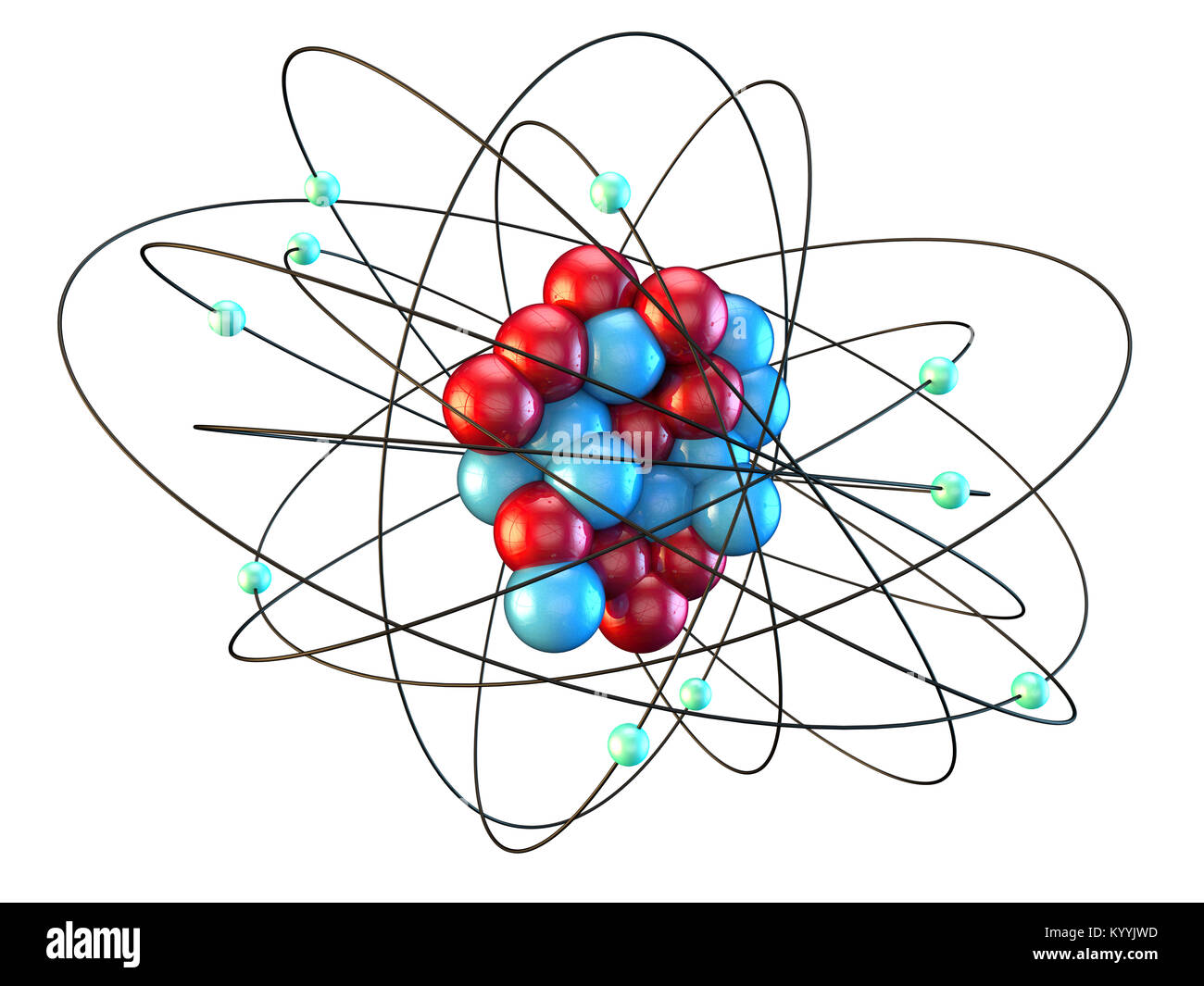
Neon atomic structure hires stock photography and images Alamy
Sir William Ramsay Gustav Hertz Related Topics: chemical element noble gas air neon (Ne), chemical element, inert gas of Group 18 ( noble gases) of the periodic table, used in electric signs and fluorescent lamps.

Atom Neon 3D model Chemistry Projects, Science Projects, School Projects, Projects For Kids
Neon is a colorless, odorless, tasteless gas. It changes from a gas to a liquid at -245.92°C (-410.66°F) and from a liquid to a solid at -248.6°C (-415.5°F). Its density is 0.89994 grams. A man bends a glass tube that will be used for neon lighting. The completed, glowing tubes are in the background.

Neon Atom on White Background Stock Illustration Illustration of physics, scheme 51926743
Help & legal Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

Neon Atom Science Notes and Projects
Just the facts. Atomic number (number of protons in the nucleus): 10. Atomic symbol (on the Periodic Table of Elements ): Ne. Atomic weight (average mass of the atom): 20.1797. Density: 0.0008999.

Pinterest • The world’s catalog of ideas
The Bohr Model of Neon (Ne) has a nucleus that contains 10 neutrons and 10 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Neon contains 8 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Neon (Ne)?
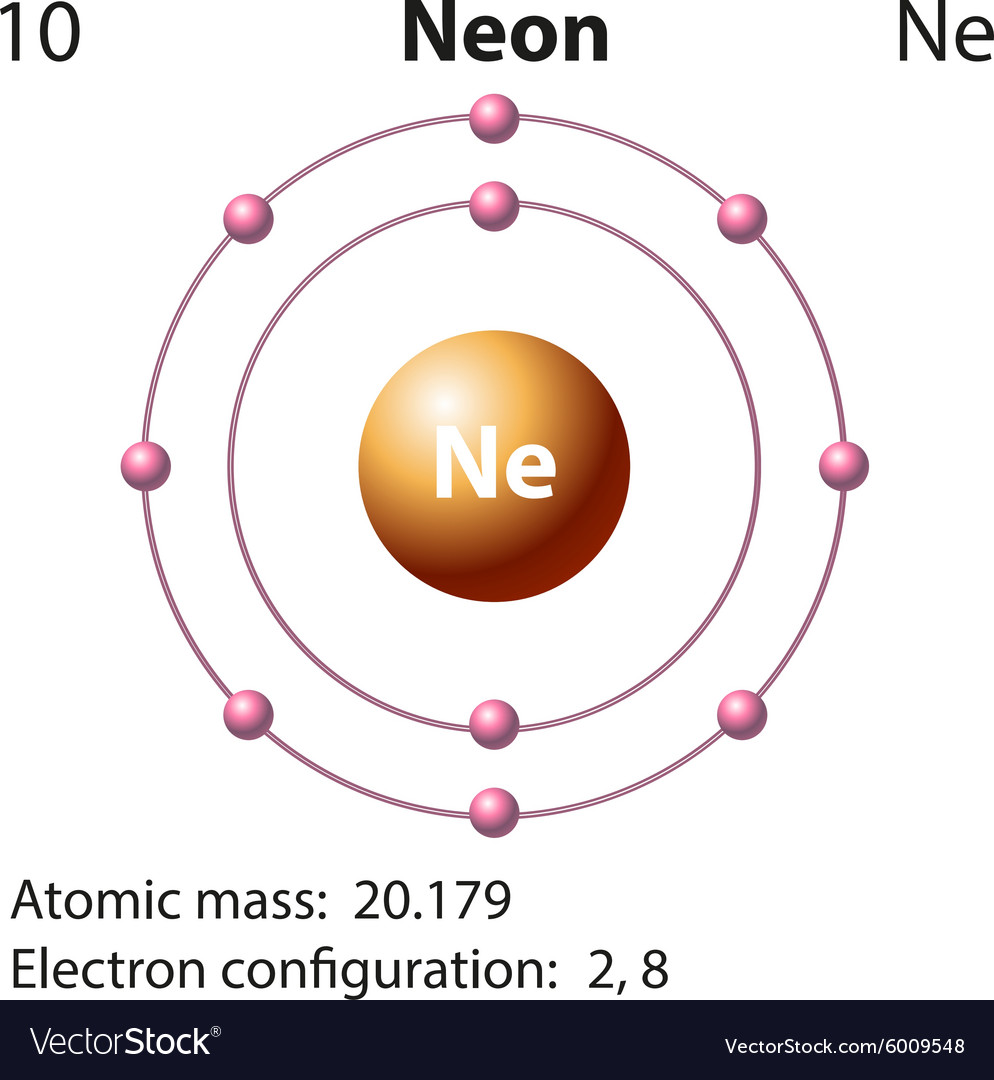
Diagram representation of the element neon Vector Image
An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885-1962).. neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they would be more energetically stable if they followed the octet.

STRUCTURE OF AN ATOM Neon atom, Neon atom model, Atom model
Neon was discovered by Sir William Ramsay, a Scottish chemist, and Morris M. Travers, an English chemist, shortly after their discovery of the element krypton in 1898. Like krypton, neon was discovered through the study of liquefied air. Although neon is the fourth most abundant element in the universe, only 0.0018% of the earth's atmosphere is.

How to Make a Model of the Neon Atom Sciencing
Atomic Structure (Bohr Model) for the Neon (Ne) Atom Wayne Breslyn 718K subscribers Join Subscribe Subscribed 35 Share 5.7K views 1 year ago In this video we'll look at the atomic structure.
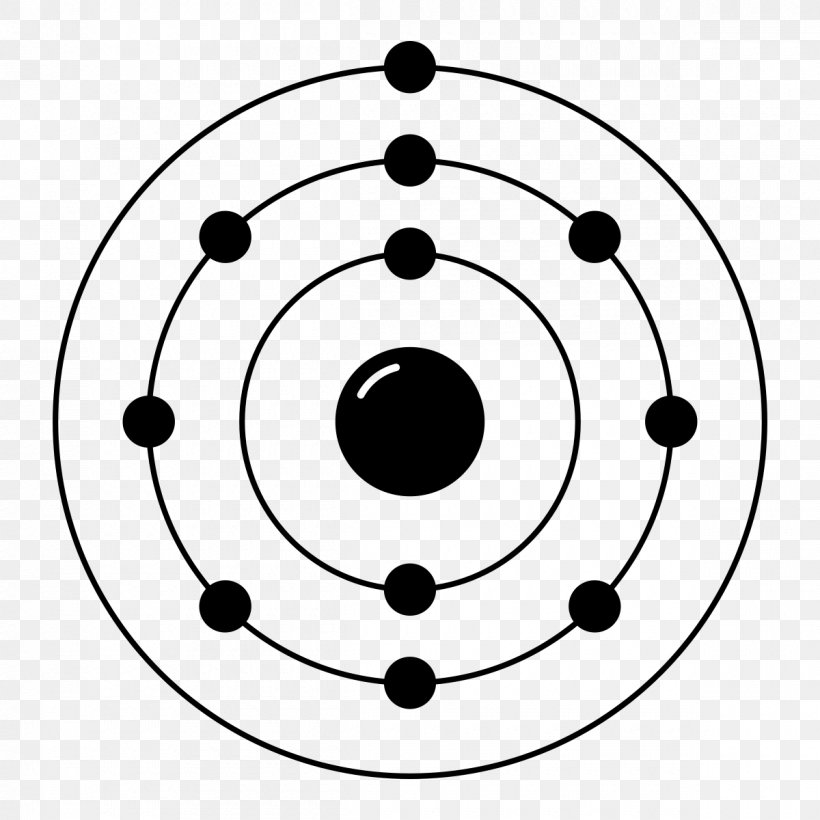
Neon Electron Configuration Bohr Model Electron Shell Noble Gas, PNG, 1200x1200px, Neon, Area
Neon (Ne). Diagram showing the nuclear composition and electron configuration of an atom of neon-20 (atomic number: 10), the most common isotope of the element neon. The nucleus consists of 10 protons (red) and 10 neutrons (blue). Ten electrons (green) bind to the nucleus in shells (rings), filling the outer (second) electron shell in what is a.

Image result for neon atom model Science Projects For Kids, Science For Kids, Craft Projects
Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.. This is the source of light emitted by neon signs and is also the source of light in a fire. Figure \(\PageIndex{1}\): Human/Need/Desire. Neon sculpture by Bruce Nauman (1983.

3d render of atom structure of neon isolated over white background Protons are represented as
Neon element is the 4 th most abundant element found in the entire universe. The name "neon" came from the Greek word "novum", which means new. Neon gas is lighter than air and its density is ⅔ rd the density of the air. Neon is the second lightest noble gas in the noble gas family. (Helium is the lightest of all the noble gases.)

Some Syrup with my Waffle Connor's Atom Project Neon
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.. Similarly, neon has a.
Visualizing Chemistry Activity 2 Atom and Atomic Structure
Here's how you can draw the Bohr model of neon step by step. #1 Write protons, neutrons, and electrons of neon atom. #2 Draw nucleus of neon atom. #3 Draw 1 st electron shell. #4 Draw 2 nd electron shell. Let's break down each step in detail.